23-Jul-2021
GABAA receptors are ligand-gated chloride channels in the vertebrate central nervous system, with a well-documented pharmacology: GABAA receptors are the targets of several human drugs, with anticonvulsant, anti-anxiety, analgesic, sedative and anaesthetic properties.
Despite the clinical importance of the GABAA receptor, its structure and functionality are not clear. Its human drug modulators were in use for decades before it became clear that it was GABAA which was the target.
Until recently, our understanding of the binding and conformation of GABAA was inferred from the study of homologues in invertebrates, and physiologically dissimilar ion channels in humans. However, Cryo-EM reconstructions of the GABAA receptor has previously resulted in “collapsed” conformations, suggesting that the structure of GABAA was different from typical ligand-gated ion channels, and with GABAA homologues.
Recently, two studies managed to solve the structure of full-length human α1β3γ2L GABAA receptors, utilising structural biology methods from the Instruct Centre BE – Nanobodies4Instruct facility.
Laverty et al (2019) used megabodies to present the receptor’s structure “functionally reconstituted in lipid nanodiscs”, whilst Masiulis et al (2019) presented the receptor bound in 5 different complexes.
Megabodies are built from nanobodies which are then bound to larger scaffold proteins – designed to help align single particle images in cryo-EM. Laverty et al (2019) utilised megabodies (specifically Mb38) to randomise GABAAR orientation in ice and facilitate particle alignment – thus ensuring the structure could be solved.
This study presented the most complete high-resolution structure of GABAAR in situ in a lipid bilayer (Fig 1). It expands on previous attempts to solve the structure in the presence of detergents, which had impacted inter and intra-subunit interfaces. The results open avenues for future functional and structural studies of GABAAR modulation by lipids and small molecules that target the cellular transmembrane domain.
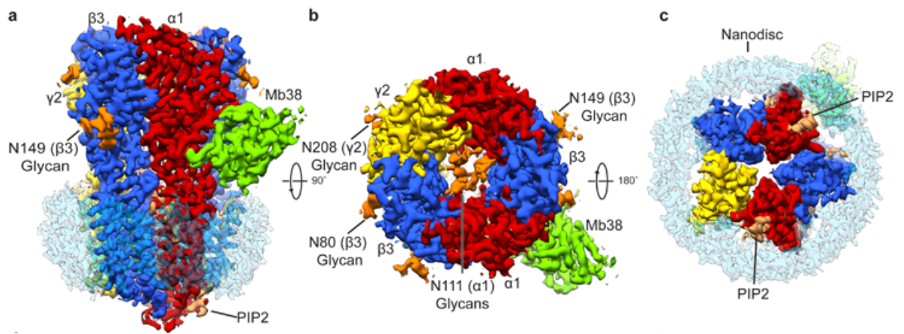
Figure 1. Side (a), top (b) and bottom (c) views of the sharpened cryo-EM map of α1β3γ2L GABAAR-Mb38 complex in lipid nanodisc. Mb38 and glycans are colored green and orange, respectively.
The study by Masiulis et al (2019) sought to gain a better understanding of how GABAAR interacts with various modulators, including channel blockers, agonists, antagonists, and two pharmaceutical drugs.
Picrotoxin (PTX) is traditionally used as a “channel blocker” for GABAAR. However, results from the study surprisingly found that it, in fact, inhibits GABAAR by binding to an allosteric site, closing the channel pore and preventing the binding of the GABA neurotransmitter. By solving the structure of the closed pore using cryo-EM (and, again, using Mb38 megabodies), the researchers found that PTX acts as an allosteric competitive inhibitor of GABAAR. They found that bicuculine (BCC) also acts as a competitive inhibitor, but instead binds to the agonist GABA binding site and stabilises GABAAR in a closed state.
GABA-bound structures in comparison to agonist-free structures indicate how the agonist works, by rotating the extracellular domain (ECD) and ensuring the channel pore remains open.
The study also examined the effects of traditional pharmaceutical drugs on the receptor, in this instance the benzodiazepines (BZDs) alprazolam (Xanax) and diazepam (Valium). The study found that these bind to the ECD and facilitate the rotation required to open the channel pores (Fig 2). However, prolonged exposure to GABA and BZDs will lead to total desensitisation of the pore.
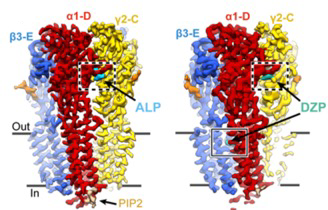
Figure 2. The cryo-EM map of the α1β3γ2 GABAAR in complex with alprazolam (cyan) and diazepam (teal) viewed parallel to the membrane plane.
This study again outlines the strength of cryo-EM in studying and solving protein structures in human membranes, and specifically in the design of safer and more specific anxiolytic, sedative, hypnotic and anticonvulsant drugs.
Both studies provide a far deeper insight into the structure and function of GABAAR than previously discovered, and the importance of structural biology in making significant strides in healthcare and pharmaceutical development.