03-Aug-2022
The group II chaperonin TRiC (TCP-1 Ring Complex) is essential for the folding and function of a wide variety of proteins including the cytoskeletal polypeptides, actin and tubulin.
As previous studies have uncovered, TRiC is made up of 8 subunits, CCT1-CCT8 (chaperonin containing tailless complex polypeptide 1), folded into a hexadecamer of two back to back rings, forming a folding chamber which binds to the nascent proteins. The structure also supports binding and hydrolysis of ATP, which drives closure of the “lid”, and switching TRiC to its closed state. However, although the structure of the TRiC complex is now well known, understanding of the events that occur once a nascent protein is confined within the chamber has previously been limited.
Kelly et al. (2022) aimed to gather insights into the process of folding proteins by human TRiC, using Cryo-EM at two Instruct Centres (UK and Finland), entrapping TriC-bound substrates along the folding cycle, to examine the closed conformation of TRiC, and to see its interaction with key substrates.
Human endogenous TRiC complexes associated to their substrates were purified and analysed using Cryo-EM. In order to study the folding process in the TRiC chamber, a transition-state mimic was used to trap the TRiC complex in a folding-competent state. In collaboration with Instruct Centre Belgium, a nanobody specific to TRiC subunit CCT5 (Nb18) was also raised and added to the TRiC complexes in order to aid subunit alignment at the moment of Cryo-EM data processing.
The Cryo-EM work was carried out at Instruct-FI, at the University of Helsinki, and also at Instruct-UK, at eBIC at Diamond Light Source, and at the Oxford Particle Imaging Centre. Furthermore, the Cryo-EM data was analysed by Scipion, the software developed by Instruct-ES, I2PC.
The consensus map of closed TRiC particles showed additional density within the folding cavity, corresponding to TRiC substrates - the most abundant of these substrates being β-tubulin peptides. The team found that the N- and C-terminal domains of β-tubulin contacts several of the CCT subunits on the rings, most prominently with CCT3 and CCT6, while the largely disordered taxol-binding domain remains in the cavity centre. This work was a first look at β-tubulin folding intermediates with folded regions of tubulin held against the inner wall of TRiC (Figure 1A), which allows unfolded regions to conform to their structure in the chamber itself.
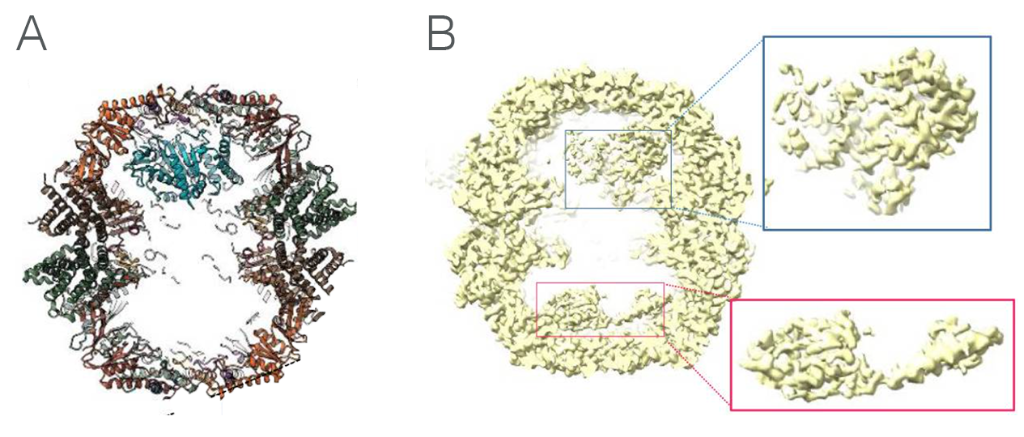
Figure 1. A: TRiC-tubulin complex, showing how the tubulin polypeptide contacts with the inner wall of TRiC at one end, allowing unfolded areas to achieve their native state in the chamber itself. B: TRiC-actin complex showing actin bound to the inner walls of both chambers. Folding is assisted in the chambers by PhLP2A co-chaperone, which was not modelled.
TRiC-actin complexes were also found, albeit to a slightly lesser extent than tubulin (20% compared to 230% of all closed particles). More importantly though, actin was found to bind in combination with a co-chaperone, namely one of the four PhLPs (phosducin-like proteins) produced by humans that is known to regulate TRiC-dependent actin and tubulin folding. The study of the ternary complex structures indicates that this co-chaperone acts as a “strut” anchored to one of the cavities, whilst stabilising actin folding intermediates in the other cavity.
Interestingly, the study by Cryo-EM of the TRiC-substrates structure in an open state reveals different binding sites, indicating that important changes in the TRiC-substrate interactions occur throughout the folding process.
What these complexes told the team was that unfolded polypeptides, such as actin and tubulin, are recognised at the surface-exposed apical domain of nucleotide-free TRiC and are released into the chamber by the ATP-induced lid formation. Specific contacts to subunits stabilise the already structured regions of substrate proteins, which provides the framework for the less folded regions to reach their final structure.
The importance of efficient actin and tubulin formation cannot be understated (for example, mutations that affect how they bind to TRiC are associated with congenital myopathies). Understanding how TRiC binds to actin and tubulin can therefore have profound effects on our managing of these diseases.
Read the full text of the article here: Kelly et al., Snapshots of actin and tubulin folding inside the TRiC chaperonin. Nature Structural & Molecular Biology (2022). 10.1038/s41594-022-00755-1