25-Oct-2024
Researchers at the Institut de Biologie Structurale have made significant progress in understanding NADPH oxidases (NOX), enzymes critical for producing reactive oxygen species (ROS) used in immune defence and cellular signalling. While much has been learned from eukaryotic NOX, there has been a gap in understanding how these proteins activate electron transfer, a crucial aspect of their function. A recent study on SpNOX, a homolog from Streptococcus pneumoniae, sheds new light on this process and positions SpNOX as a robust model for studying NOX enzymes, including the human NOX2.
NOX enzymes are known for their role in generating ROS, important for the immune system and signalling pathways in humans, animals, and plants. Despite structural data on human NOX isoforms, the exact mechanism of electron transfer activation remained unclear. In this new study, researchers used the automated X-ray crystallography pipelines at EMBL Grenoble to explore the structure of SpNOX, a bacterial homolog of NOX2. This access was funded by the grants iNEXT Discovery and Fragment Screen funded by the European Commission and through Instruct-ERIC. The published findings offer a deeper understanding of how electron transfer occurs within NOX proteins.
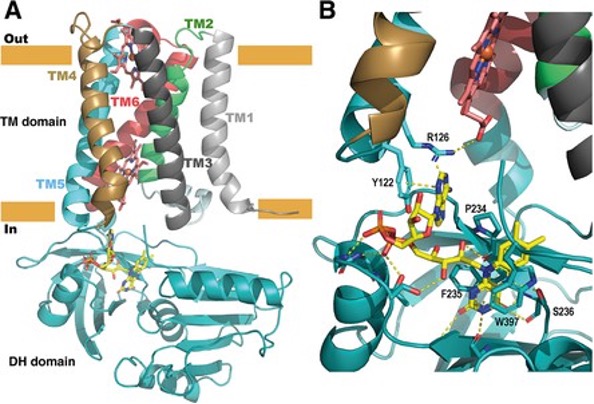
Figure 1. Structure of the full-length SpNOX. (A) Overall structure of SpNOX F397W at 3.6 Å with ribbons in the TM colored by helix, hemes (red sticks) and FAD cofactor (colored by atom). (B) The FAD binding site
Using functional and structural data, this study shows that SpNOX’s electron transfer starts with a hydride transfer from NAD(P)H to the flavin adenine dinucleotide (FAD) cofactor, a rate-limiting step in NOX activity. Structural analysis reveals that key amino acids, such as F397, play a vital role in stabilising the transfer process. Interestingly, SpNOX can utilise both NADPH and NADH as electron donors, unlike eukaryotic NOX enzymes.
These findings highlight the significance of the hydride transfer step and suggest that internal rearrangements within the enzyme’s dehydrogenase domain are essential for activation. Comparisons with human NOX2 reveal that SpNOX could serve as a model for understanding the activation process of human NOX2, particularly in immune cells like neutrophils. The structural similarities between SpNOX and NOX2 suggest that SpNOX can help researchers uncover mechanisms crucial to NOX2 activation.
The study also complements recent work using cryo-electron microscopy (cryo-EM) to investigate NOX enzymes, further validating the structural insights provided by SpNOX. These combined efforts provide a comprehensive view of the structural and functional relationships that govern electron transfer in NOX enzymes.
This research offers promising avenues for further exploration of NOX enzymes, potentially aiding the development of therapeutic strategies targeting diseases related to oxidative stress and immune response, where NOX enzymes play a crucial role. The newly characterised SpNOX model could also facilitate future studies aimed at dissecting the structural dynamics of NOX family proteins.
Researchers believe that SpNOX will continue to be a valuable model for unravelling the molecular features and activation mechanisms of NOX enzymes, especially as new high-resolution structures are published. This discovery offers a breakthrough in our understanding of NOX enzymes, promising to advance the fields of immunology, cell biology, and disease treatment.
Read the full paper here.