09-Oct-2023
Drug-resistant bacteria presents one of the biggest challenges for medical scientists worldwide. Part of the problem has been a distinct lack of new antibiotics added to the existing arsenal. Historically, many antibiotics were developed from natural scaffolds found in soil cultures; a team from Utrecht University, part of Instruct-NL, have returned to this method to uncover new, highly-effective, non-toxic antibiotic candidates.
Last year, the group led by Markus Weingarth uncovered teixobactin, which blocks cell wall development in bacteria, making it a prime antibiotic candidate (Shukla et al, 2022). Now, they have uncovered another potential antibiotic candidate: clovibactin (Shukla et al, 2023).
Clovibactin exhibited antibacterial activity against several antibiotic resistant pathogens, including methicillin-resistant S. aureus (MRSA). Clovibactin is a depsipeptide, similar to texiobactin – it has a depsi-cycle which can bind to its bacterial targets.
To investigate the mechanism by which clovibactin killed bacterial targets, the team considered teixobactin, which induced lysis in the bacterial cell wall with mediation from AtlA, the key cell wall autolysin in S. aureus.
A test against an ΔAtlA deletion mutant found that clovibactin induces a stronger lysis than teixobactin against a wild type, but also is not heavily impacted by the ΔAtlA mutant, unlike teixobactin. This suggests that AtlA is not the only mediator of clovibactin-induced cell lysis.
To test the cell target, clovibactin was incorporated into the major biosynthetic pathways of S. aureus: DNA, RNA, protein and peptidoglycan. It specifically interfered with N-acetylglucosamine (GlcNAc) into the cell wall. Clovibactin was also used to treat B. subtilis, and found cell wall biosynthesis was also interrupted, and cell-shape deformations were observed. To narrow down the target further, lial-lux induction was monitored – an increase would indicate that clovibactin may interact with the cell wall lipid II biosynthesis pathway. The study was positive, and found that clovibactin interferes with the pathway in a similar manner to vanomycin; producing a build-up of the Lipid II building blocks in the cell cytoplasm.
ssNMR was used to determine that clovibactin and lipid II in membranes forms a well-defined complex, in which clovibactin immobilises (a similar action to teixobactin in a supramolecular structure). This fibrillar supra-structure between clovibactin molecules is predominantly mediated by backbone-backbone interactions between N-terminal residues. Atomic force microscopy showed that fibrils would form on membrane surfaces with high concentrations of lipid II and clovibactin. This rapid oligomerisation is central to the action mode of clovibactin.
ssNMR data indicated that the depsi-cycle of clovibactin binds directly to the lipid II pyrophosphate (PPi) group. This was corroborated by binding of clovibactin to lipid I and to C55PP, which lacks some of the other key structural components of lipid II. The backbone of clovibactin coordinates the binding of PPi to the depsi-cycle, shown in Figure 1.
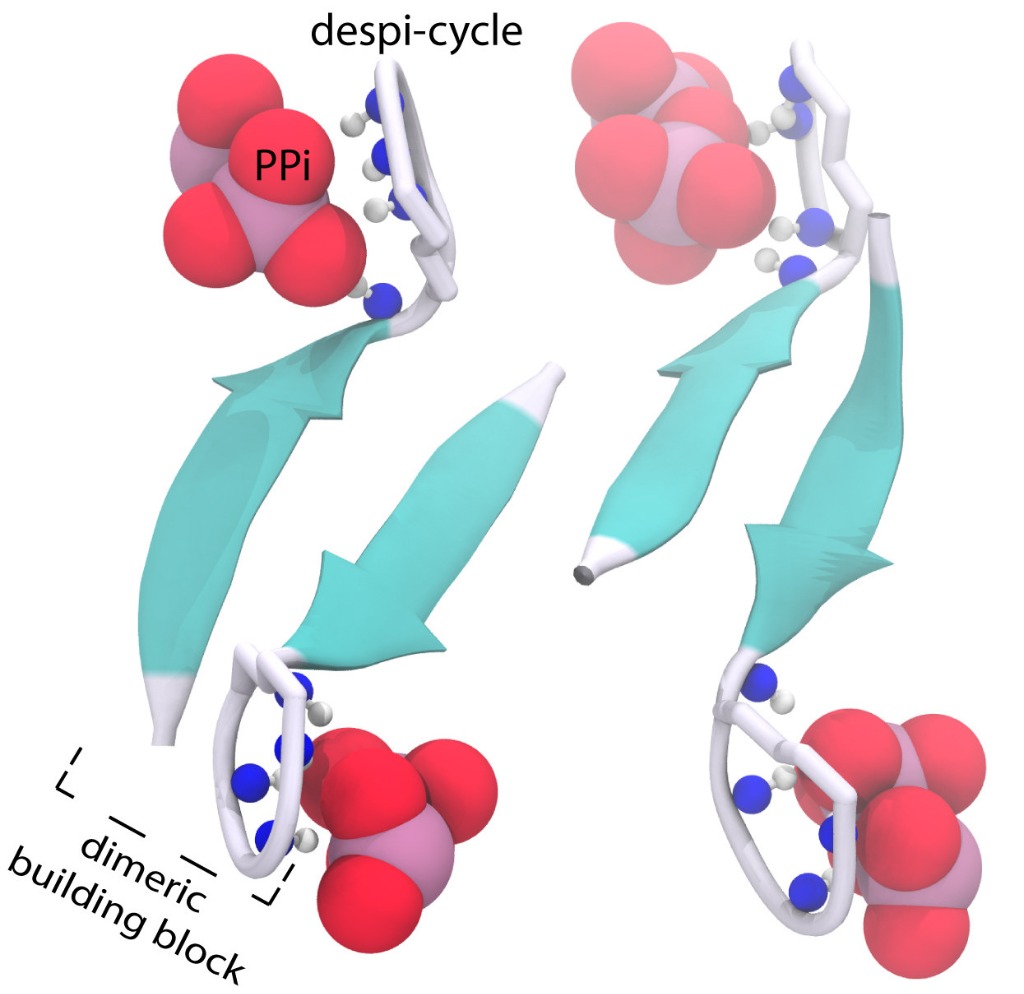
Figure 1. The structure of the clovibactin (blue) depsi-cycle (grey) in complex with the lipid II PPi group (red).
Furthermore, one of the two lipid II sugars, MurNAc, bound with almost all clovibactin depsi-cycle residues. The interface of clovibactin and lipid II, then, takes place between the depsi-cycle, and the lipid II MurNAc sugar and adjacent PPi group. However, the PPi group is considerably more prominent in clovibactin-lipid II binding, particularly compared to teixobactin (which specifically interacts with the MurNAc lipid II sugar via hydrogen bonds, while clovibactin only engages in unspecific hydrophobic interactions with the MurNAc sugar.
This lack of specific interaction between clovibactin and lipid II sugars is what allows clovibactin to bind effectively with many cell wall precursors, with a lipid-anchored PPi group. The presence of different sugars does not impact clovibactin’s affinity.
To complement the high affinity binding of clovibactin to bacterial cell walls, as well as its low resistance frequency (estimated to be 10-10, far lower than the desirable 10-8), clovibactin was also found to display zero cytotoxicity against mammalian cells at high concentrations. All in all, clovibactin looks to be a strong candidate for drug development in the ever more desperate world of antibiotics, and could have significant impacts for the fight against drug-resistant bacteria down the line.