Instruct-IL Project on the Impact of Multiple Factors in Studying the Crystal Structures of Ligand-Phosphotriesterase Complexes
01-Dec-2023
X-ray crystallography is one of the most widely utilised techniques for structure-based drug design, producing electron density maps of protein-ligand complexes that permit an understanding of the interactions involved in the formation of the complex. However, a number of factors may preclude the formation of the desired ligand-protein complex in the crystals.
The team at the Weizmann Structural Proteomics Unit,
which is part of Instruct-IL, studied complexes of Brevundimonas diminuta phosphotriesterase (PTE) with organophosphates (OPs) to assess the influence of these factors, which will be referred to below. PTEs are enzymes that are capable of rapidly hydrolysing toxic OPs such as insecticides. Wild type B. diminuta PTEs contain two Zn2+ ions in their active site, which are crucial for both ligand binding and enzymatic activity. These findings were report in the November 2023 issue of Acta Crystallographica Section D Structural Biology (
Dym et al. [2023] "The impact of molecular variants, crystallization conditions and the space group on ligand–protein complexes: a case study on bacterial phosphotriesterase" Acta Cryst D Structural Biology D79, 992-1009).
The present study involved three variants of B. diminuta PTE: A53, C23, and C23M, all three of which degrade the target OPs much more effectively than the wild type PTE. Overall, 12 crystal structures were analysed, including the apo variants of A53, C23, and C23M, as well as several complexes with phosphonate ligands.
The team examined these PTE variants under several crystallisation conditions, in order to assess the factors that affect formation of crystals of the OP-PTE complexes. These included:
• Different crystallisation conditions
• The presence of residual tags
• Differences between space groups
• The choice of ligands bound to each variant
The data obtained highlight the difficulties that may arise in general in obtaining crystals of a desired ligand-protein complex.
Crystallisation Conditions
Three different crystallisation conditions were used:
• Polyethylene glycol (PEG) 6000 and 2-methyl-2,4-pentanediol (MPD)
• Polyacrylic acid (PAA)
• Glycerol and ammonium sulphate (AS)
Presence of Zn2+ in the active site
The initial crystallisation conditions for A53 and C23 did not include excess Zn2+, and high concentrations of Tris, >50 mM, were used during their purification. In the crystal structure of A53, (A53_1), only one Zn2+ ion was observed in its active site, contrasting with the two ions present in the WT PTE. Additionally, A53_1 did not exhibit a canonical WT PTE dimer. The presence of Tris has been shown to reduce the effective concentration of Zn2+ ions in solution. To counter this effect, ZnCl2 was added during protein expression, purification, and crystallisation, so as to negate the impact of Tris. This is a common problem when working with metal ions in crystallographic studies. Interestingly, although the initial C23 purification and crystallisation conditions were virtually identical to those for A53_1, the resulting crystal structure (C23_1) displayed a canonical WT-like dimer with two Zn2+ ions in each subunit.
Thus, one needs to be careful in the purification process, so as not to deplete the desired concentrations of Zn2+.
Presence of crystallisation precipitants and cyclic compounds in the active site
Polyacrylic acid (PAA) was employed as a precipitant to crystallise various PTE variants, but none of them formed complexes with OPs. This can be attributed to the presence of acrylic acid (AA) in their active sites.
When a mixture of AS and glycerol was employed as the precipitant, the resulting crystals of A53_2 and C23M_1 revealed unidentified 6-membered rings within their active sites. Similarly, the use of PEG 6000 and MPD as precipitants led to the formation of crystals of A53_3 which displayed an unidentified 6-membered ring within its active site. As in the crystals obtained with PAA as the precipitant, these rings bind to the Zn2+ ions, thereby preventing binding of the OPs.
Thus, careful precautions need to be taken in selection of the crystallisation conditions so as not to prejudice binding of the OP ligands.
Residual Tags and Space Groups
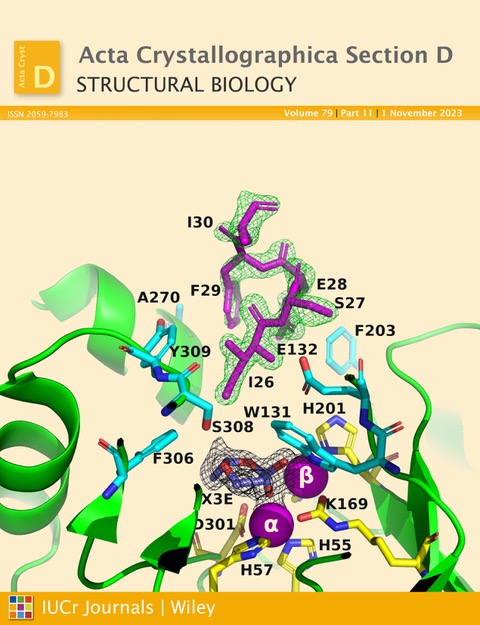
Each variant was expressed either without a fusion protein or tag, or as a fusion protein with an NH2-terminal maltose-binding protein (MBP) tag. MBP was fused to PTE via a factor Xa cleavage sequence, followed by an eight amino acid residue spacer. It was found that in crystals of A53_3 and A53_4, which were the only two crystals that formed in the P21 space group, the octapeptide spacer of the B subunit penetrates the active site of subunit A, and thus might be expected to interfere with the binding of OPs.
This may indeed explain why OPs do not form complexes with these variants in the corresponding crystals.
Figure 1. Ribbon representation of the A53_3 variant. It shows the octapeptide tag on subunit B penetrating the active-site region of the symmetry-related subunit A. The tag is shown in magenta. The active-site residues of the symmetry-related chain A are shown in yellow, with those residues within 5 Å of the tag shown in cyan. The green electron density corresponds to an omit map with the octapeptide omitted (contoured at 3). The cyclic compound X3E, which was presumably carried over from the protein expression and purification process, is seen in the active site, and the black electron density corresponds to a 2Fo-Fc map (contoured at 1). The two Zn2+ ions are shown as magenta spheres. (Figure featured on the cover of the November 2023 issue of Acta Crystallographica Section D Structural Biology).
Visualisation of OP ligands within the active site of PTE
Four different OP ligands were synthesised for use in this study: Methylphosphonic acid, O-ethyl methylphosphonic acid, ethyl-O-(N,N-diisopropylaminoethyl) methylphosphonate, and O-isopropyl methylphosphonic acid.
A commonly employed procedure for obtaining crystals of ligand-protein complexes is to soak the ligand into native crystals. This requires the ligand-binding site to be accessible to the ligand in the soaking medium. Unfortunately, in the case of the PTE variants that were studied this approach failed, since their active sites were already occupied, either by the octapeptide linker, by crystallisation precipitants, or by unidentified six-membered rings, all of which bound more tightly than the OPs, which could not, therefore, displace them.
Co-crystallisation was, therefore, employed instead. But this approach was only successful for crystals formed in AS and glycerol. For all three variants, A53_5, C23_3, and C23M_2, methylphosphonic acid was able to replace the six-membered ring present in the ligand- binding site. In the crystal structures thus obtained, the oxygen atoms of the OP are seen to be in close contact with the Zn2+ ions in the active site.
Co-crystallisation with other ligands was, unfortunately, not as effective. Since methylphosphonic acid possesses an extra P-O bond compared to the other ligands tested, it has a greater negative charge, which likely enhances its affinity for the Zn2+ ions within the active site of PTE.
This confirms the contribution of negative charge density to association of the OPs with the PTE binuclear centre.
Summary
The present study emphasises the multitude of factors that must be considered when attempting to obtain crystals of ligand-protein complexes suitable for successful X-ray data collection. These factors include the molecular construct, the selection of purification tags, the reagents employed in crystallisation, and the space groups in which the crystals form.
It can thus serve as a guide for minimising the risks associated with structure-based drug design which relies on the crystal structures of ligand-protein complexes, and to avoid falling into the common traps outlined above.