View All Electron Microscopy at Instruct
The facility will provide support and expertise to researchers using different techniques: Cryo-EM sample preparation and characterisation, Cryo-EM image acquisition of unstained biological material for cryo-EM single particle or cryoelectron tomography (cryo-ET), cryo-electron diffraction (microED) and cryocorrelative microscopy (cryo-CLEM). For many of these services, it is advisable to contact the Instruct Image Processing Centre for data processing support).
Based at the premises of the Centro Nacional de Biotecnología (CNB), the CSIC cryoelectron microscopy (CryoEM) facility is a state-of-the-art core service that offers both sample preparation and CryoEM image collection of unstained biological material.
The facility hosts a 200 kV cryoelectron microscope FEI TALOS Arctica, equipped with an autoloader and a Falcon 4i direct electron detector. Both are suited for the collection of large amounts of high-resolution data that can be used for high-resolution studies using single-particle methodology, or for the reconstruction of pleiotropic structures such as cells and subcellular structures using cryoelectron tomography (cryoET). Additionally, the facility hosts a standard 120 kV electron microscope JEOL JEM1400 for sample screening.
Specimen vitrification is also available at the service, with two devices suited for vitrification of small specimens such as proteins and macromolecular complexes (a FEI Vitrobot and a Leica EM CPC), and a third device suited for the fast-freezing of cellular samples, a Leica EM GP2 high-pressure freezer. This facility is unique in Spain as it also offers the newly developed cryocorrelative microscopy technique, which allows the analysis of the same biological specimen by cryooptical microscopy (Zeiss LS900 AiryScan cryoconfocal microscope) and cryoelectron microscopy (both cryoelectron microscopes described above). The use of a Zeiss CrossBeam 550 cryo-FIB-SEM microscope will increase the cryocorrelative microscopy capabilities of the facility to direct visualization of cells for tissue-cell resolution or for preparation of thin lamellas in cells for molecular resolution.
Vitrified cellular or tissue samples on grids or planchettes will be analysed by cryoconfocal microscopy (Zeiss LS900 AiryScan) to gain functional and location information using a Linkam stage. Analysed samples will be transferred to a Zeiss CrossBeam550 microscope together with the fluorescence information collected to generate, in the selected areas, lamellas for cryoelectron tomography (this could be done in our facility), or alternatively cryo-volume imaging. Samples could be provided by the user or from a previous project in our facility of cryoCLEMsample characterisation.
It is extremely important to contact the facility (cryoemcsic_facility@cnb.csic.es) before submitting the proposal, to address the technical viability of your experiment and discuss the details on how they should be done.

The facility will provide support and expertise to researchers in the optimisation of cellular or tissue sample preparation for cryoCLEM workflow including plunge freezing (FEI Vitrobot Mark IV, Leica EM CPC and Leica GP2 ) and high pressure freezing (Leica ICE). Once vitrified, sample screening and selection will be performed by cryofluorescence microscopy using a Linkam stage coupled to either a widefield or confocal microscope (Zeiss LS900 AiryScan).
It is extremely important to contact the facility (cryoemcsic_facility@cnb.csic.es) before submitting the proposal, to discuss the technical feasibility of your experiment and discuss the details on how it will be carried out.
a) Single particle High-end data acquisition. High-quality cryogrids could either be provided by the user or come from a successful sample characterisation project.
b) Cryo-EM tomography Data acquisition. Cryo-grids could be provided by the user or from a previous project in our facility of sample characterization (it is advisable to contact the Instruct Image Processing Centre for support in the data processing).
Micro-Electron Diffraction data are acquired in a Talos Arctica 200 kV microscope equipped with a scintillation-based Ceta-D camera optimised for low dose data acquisition, ideal for projects involving dose-sensitive samples such as proteins, peptides, pharmaceutical compounds and organic materials.
Basic workflow for sample handling and data acquisition:
Mandatory information:
If previous diffraction experiments have been performed, a very short description of the results: problems, unit cell, space group, crystal shape and size.
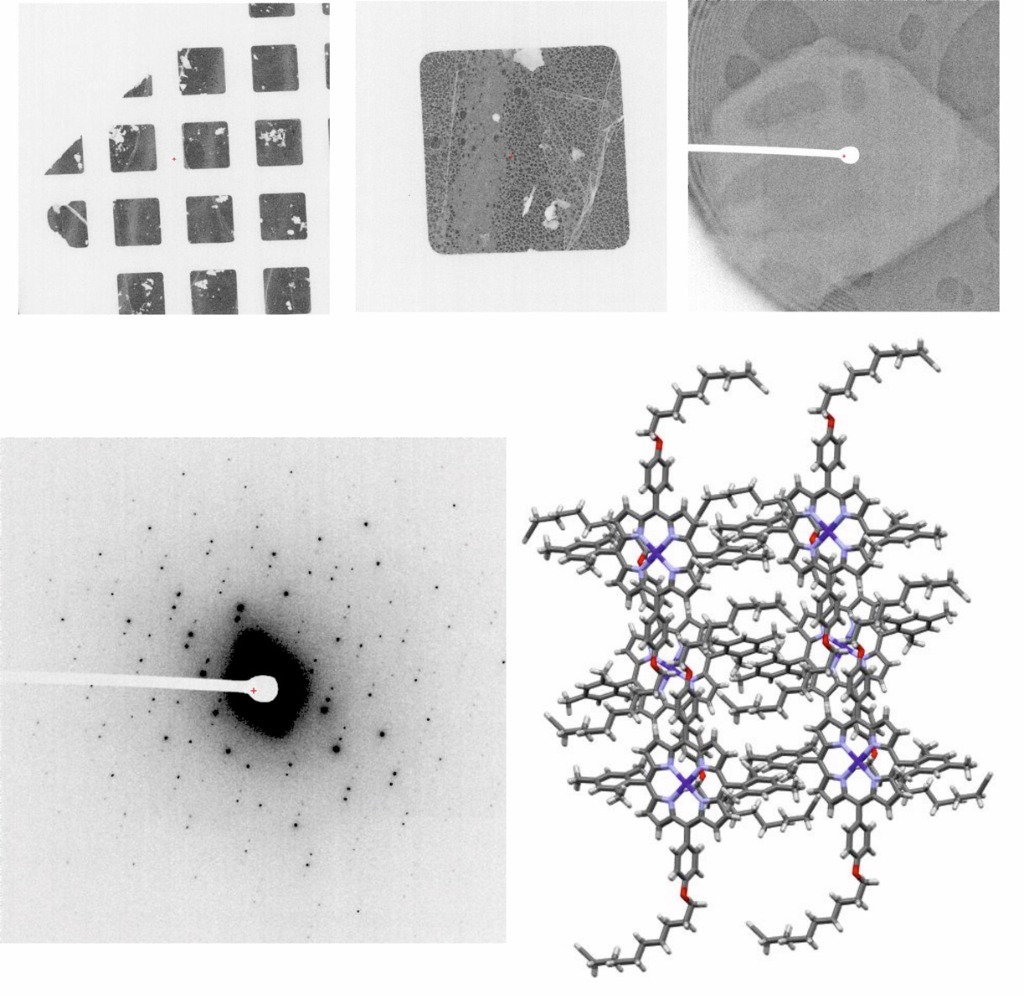
The facility will provide support and expertise to researchers with samples in the first stages of characterisation. Samples will be analysed by negative staining in search of the best conditions. Once found, samples will be subjected to different vitrification conditions in search of the best parameters, which will be tested in a cryomicroscope Talos Arctica 200 kV equipped with a Falcon 4i electron direct detector. The best grids will be used to acquire some data for image processing, to check the real quality of the sample (it is advisable to contact the Instruct Image Processing Centre for support in the data processing).
In order to provide you with our best services, we require the following information: