Neisseria meningitidis is the bacterial pathogen responsible for meningitis and septicaemia. Previously, a polysaccharide-based vaccine has been developed against Meningitis A, C, Y, W-135. However, the capsular polysaccharide of Meningitis B is a structural mimic of a human cell adhesion molecule, making it poorly immunogenic. As such, the development of Meninigitis B vaccines has required a novel approach.

• The 3D structures of some fHbp variants were determined by NMR spectroscopy and the interactions with antibodies were analyzed to describe the epitopes for the various Meninigitis B strains.
• A large number of surface mutants were designed to inserting epitopes for the different variants on a single antigen scaffold.
• The chimera antigens were assessed with respect to bactericidal activity and to their folding and structural stability by NMR. The most effective chimera protein was chosen for further implementation.
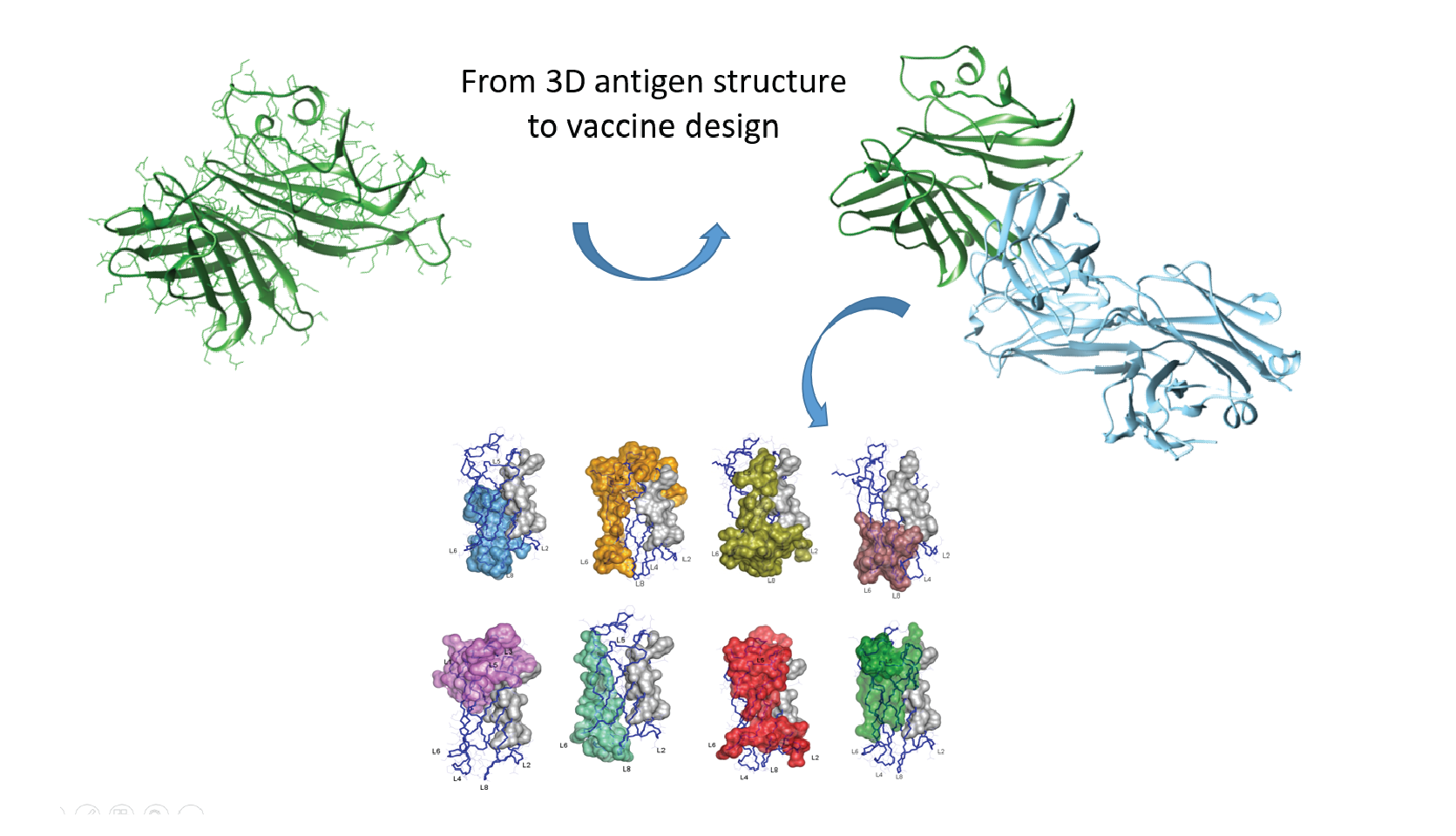
Through atomic-level structure determination, researchers have successfully engineered a single molecule of fHbp that induces protective immunity against all its antigenic variants. The detailed knowledge of the structural properties and of the antigen-antibody interactions contributed to vaccine approval. The development of a structure-based approach is expected to facilitate the design of vaccines against a variety of pathogens with high antigenic variation.

The collaboration between our company and the Italian centre of Instruct-ERIC has been key for determining the structure-based antigen design against Neisseria meningitidis serogroup B using NMR spectroscopy. Thanks to the high-end NMR instruments and the expertise in structural biology available at CERM/CIRMMP, we have been able to deliver the first recombinant protein-based meningococcal vaccine targeting a challenging disease.
Dr Ilaria Ferlenghi, GSK Vaccines, S.r.l.
