 Instruct
InstructFeaturing expert speakers from Instruct Centres across Europe, Instruct-ERIC Webinar Series: Structure Meets Function highlights some of the latest developments in structural biology, demonstrating how integrative methods are enabling scientists to decipher the mechanisms that underpin health and disease.
Watch the previous webinars in the series here.
The 11th webinar in the series will be hosted by Instruct Centre France 2, on 8 Jun 2021, 11:00 - 12:30 CEST
Agenda
Webinar moderator: Darren Hart, IBS Grenoble
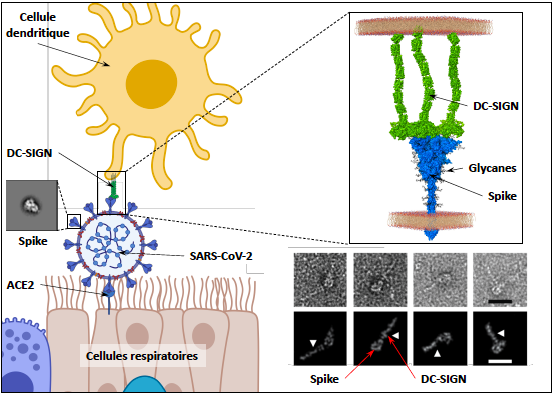
Talk 1: DC-SIGN and L-SIGN receptors from immune cells recognize spike glycoprotein and promote SARS CoV-2 trans infection
Speaker: Franck Fieschi
Affiliation: IBS Grenoble
Abstract: The efficient spread of SARS-CoV-2 resulted in a unique pandemic in modern history. Despite early identification of ACE2 as the receptor for viral spike protein, much remains to be understood about the molecular events behind viral dissemination. We evaluated the contribution of C-type lectin receptors (CLRS) of antigen-presenting cells, widely present in respiratory mucosa and lung tissue. DC-SIGN, L-SIGN, Langerin and MGL bind to diverse glycans of the spike using multiple interaction areas. Using pseudovirus and cells derived from monocytes or T-lymphocytes, we demonstrate that while virus capture by the CLRs examined does not allow direct cell infection, DC/L-SIGN, among these receptors, promote virus transfer to permissive ACE2+ cells. A glycomimetic compound designed against DC-SIGN, enable inhibition of this process. Thus, we described a mechanism potentiating viral capture and spreading of infection.
Talk 2:Photoenzyme at work: Structural dynamics of fatty acid photodecarboxylase
Speaker: Martin Weik
Affiliation: IBS Grenoble
Abstract: Photoenzymes require sunlight for catalysis. Among those, the recently discovered fatty acid photodecarboxylase (FAP) catalyses the generation of hydrocarbons from lipids, a process that bears great promise for the production of biofuels. How the enzyme accomplishes its function at the molecular level remained mysterious, however. Observing the inner workings of FAP in both space and time has been accomplished by an international consortium of scientists that combined molecular biology, biochemistry and experimental and computational methods: kinetic crystallography at the ESRF and serial femtosecond crystallography (SFX) at an XFEL, in crystallo spectroscopy at the icOS lab, time-resolved vibrational and optical spectroscopies, mutagenesis and quantum chemical calculations. In the presentation, the contribution of static and time-resolved SFX will be highlighted.
Talk 3: The high resolution cryo-EM structure of full-length La Crosse virus polymerase reveals functionally important conformational changes
Speaker: Helene Malet
Affiliation: IBS Grenoble
Abstract: Bunyavirales is an order of segmented negative-strand RNA viruses comprising several life-threatening human pathogens for which there is currently no treatment (La Crosse virus, Hantaan virus, Crimean Congo virus, Lassa virus). The replication and transcription of their genome are essential steps of their viral cycle and are catalyzed by a key viral enzyme: the RNA-dependent RNA polymerase. We describe here the structure of the complete RNA-polymerase of the La Crosse virus obtained at 3 Å resolution by cryo electron microscopy, using data collected on the Glacios from the Grenoble electron microscopy platform and on the Krios from ESRF. This structure reveals the position and organization of the C-terminal part of the RNA polymerase which includes a cap-binding domain necessary for transcription initiation. Two states could be visualized, pre-initiation and elongation. In particular, this allows to highlight the conformational changes necessary for the formation of a double-stranded 10-base pair RNA in the active site cavity during elongation. The structural details and dynamics of the functional elements identified provide mechanistic insight into bunyavirus transcription and may be crucial for the future development of RNA polymerase inhibitors.
Reference: Pre-initiation and elongation structures of full-length La Crosse virus polymerase reveal functionally important conformational change. Benoît Arragain, Grégory Effantin, Piotr Gerlach , Juan Reguera , Guy Schoehn, Stephen Cusack, Hélène Malet. Nature Communications;. 2020 Jul 17;11(1):3590.