16-Jun-2023
Treatment against SARS-CoV-2 has mostly come in the form of vaccinations and preventative measures, however these are always at risk of inefficacy by variants. In order to provide a more flexible solution, prophylactic treatments have been developed.
One such target area is the nasal epithelium, which sees much of the replication of SARS-CoV-2 before its transport to the lungs. A prophylactic solution considered here by Mäkelä et al (2023) was a Sherpabody (SH3 Recombinant Protein Affinity; targeting modules derived from the nephrocystin protein, and which can be constructed to mimic antibodies), engineered for intranasal prophylaxis against a highly conserved region of the SARS-CoV-2 spike receptor binding domain (RBD).
A protein screen found 15 unique sherpabodies which could bind strongly and specifically to the SARS-CoV-2 RBD, and one in particular could also bind to SARS-CoV-1, which suggested it might have strong binding to conserved areas of the RBD, and would be less prone to vaccine escape. This individual sherpabody was Sb92. A trimerised version (TriSb92) was constructed to more effectively target the trimeric viral surface spike protein. Other clones could also bind to SARS-CoV1, but did not have as strong binding to the SARs-CoV-2 RBD, so Sb92 was selected.
TriSb92 was incubated with different viral isolates with serial dilutions, to test its neutralising efficacy against the Wuhan and alternative strains. It was found to be effective against all variants of concern, particularly the Beta (B.1.351), Delta (B.1.617.2), and latest Omicron variants like BF.7 and XBB.
This was especially the case when TriSb92 was administered intranasally prior to SARS-CoV-2 infection, resulting in almost total protection against the virus. Additionally, therapeutic administration of TriSb92, even up to 8 hours post-infection, led to a significant reduction in SARS-CoV-2.
Insight into the molecular mechanisms underlying this strong neutralisation was determined through cryo-EM structure determination, where the cryo-EM facilities at University of Helsinki, part of Instruct-FI, were instrumental.
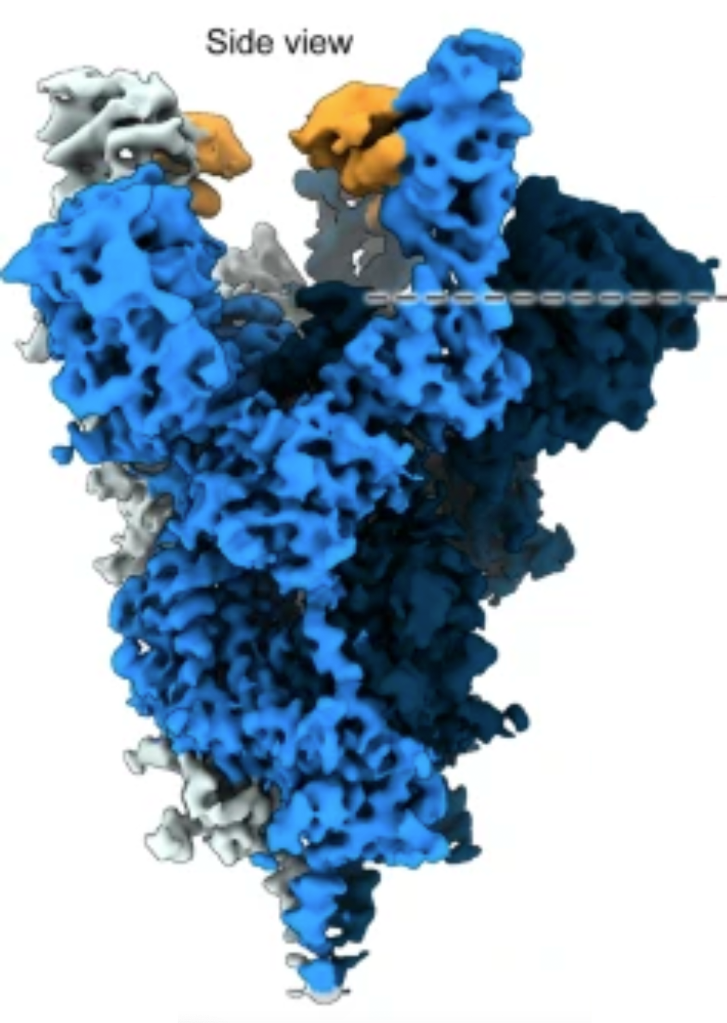
Figure 1. Spike trimer in complex with TriSb92
It was found that although TriSb92 binds to the RBD, it does not then block it from binding to ACE2, the target receptor on host cells (the mechanism of many other vaccines). Instead TriSb92 locks the RBDs in an “all up” conformation, destabilising the spike and ultimately resulting in a conformation that cannot support productive viral entry after receptor binding.
The area that TriSb92 binds to is highly conserved between SARS-CoV-2 and SARS-CoV-1, and there are minimal changes in this predicted binding site between the variants of concern.
This makes TriSb92 extremely robust to further mutations of these (or new) variants, and therefore a potent potential target for prophylactic treatment. The other benefits of TriSb92 are its ease of proliferation in high quantities with low-cost expression systems, as well as its stability in “shelf-life” temperatures.
The study clearly demonstrates that this sherpabody could be an effective non-vaccine intranasal treatment for SARS-CoV-2, and could be a “valuable addition to the arsenal of available measures” against the virus.