20-Jan-2023
Conservation of Drosophila melanogaster Septin G-interfaces studied by University Sao Paulo
Septins are filamentous GTP-binding proteins present in most eukaryotes. Their role was initially identified to be in the process of cytokinesis and septum formation in yeast, but has expanded beyond cell division to several important cellular processes. These include cell structure and polarity, exocytosis, motility, and more. The ability of septins to form structures such as bundles, rings, and gauzes is central to these processes.
Richard Garratt and colleagues from the University of Sao Paulo decided to investigate whether the G-interfaces of septins (which are crucial to septin filament formation) are conserved between Drosophila melanogaster and their human homologues.
This study was part of the Instruct-ERIC Second International Call, inviting researchers from institutions that hold an MOU with Instruct to visit European facilities for their study. The team from University of Sao Paulo accessed technology at Diamond Light Source of Instruct-UK.
Richard said, "Science is an inherently globalised endeavour and the increasing ease of access to advanced experimental infrastructure is helping to make it more democratic also. The Instruct-ERIC international calls have been fantastic in giving rapid access to Latin American scientists, like ourselves, to the necessary facilities that we need in order to try to remain competitive on the world stage."
The aim of the study was to explore the conservation of the G-interfaces of septins in D. melanogaster with their human homologues. The septins (and their homologues) in question were:
This is not merely “crystallographic stamp collecting”, as it was deemed important to understand how Drosophila forms functional filaments without expressing homologues of a fourth group of human septins (subgroup I), which greatly increases filament diversity. The Drosophila septins were purified, and their size exclusion chromatography profiles indicate several conservation notes. Sep1 and Sep4 would form a combination of monomers and dimers when expressed alone (same as their human homologue). Sep2 and Sep5 form only monomers (same as their human homologue). Pnut again only forms monomers, however this is different to its human homologue SEPT7 (which is dimeric).
The study then examined the G-interfaces of all septins in heterodimers. Co-expression of the G-domains from the different septins led to the formation of heterodimers as predicted. These heterodimers (Sep1.Sep2,Sep1.Sep5, Sep4.Sep2, Sep4.Sep5) are all bound to both GDP and GTP. In these complexes, Sep1 and Sep4 would bind to GDP, and Sep2 and Sep5 would bind to GTP – which aligns with their human homologues. However, Pnut, when expressed as a homodimer, demonstrated both GDP and GTP binding. This is interesting for the study as this is different to SEPT7, the human homologue of Pnut.
To study more deeply the conservation of the septin’s G-interfaces in both Drosophila and humans, the crystal structure of the Sep1. Sep2 heterodimer was solved, the first of any D. melanogaster septin. The structure highlighted several conserved elements with human septins. First, the specific binding interface which unites the two septins is highly conserved between D. melanogaster and humans. Furthermore, the conformational changes that occur when each septin binds to the appropriate G-domain partner is also conserved. Second, Sep1 binds to GDP and Sep2 binds to GTP. Third, similar to its human homologue, within the crystal lattice Sep1 and Sep2 also form a non-physiological NC-interface. This result, although consistent with the human structures, is nevertheless curious, as current knowledge would predict an NC interface between two copies of Sep2 instead.
Other heterodimers were assessed using AlphaFold2, examining their similarity to Sep1.Sep2. The Sep1.Sep5 binding mechanism is very similar. Sep4 has significant substitutions in the trans loop of its binding domain, however its binding to Sep1 or Sep5 remains intact. Sep5 also has an individual change to its trans loop. This change indicates it may be better suited to binding with Sep4, whereas Sep1 is more efficiently bound to Sep2.
The study also explored the differences between Pnut and SEPT7. It found that the chief difference was a lack of electrostatic interactions in Pnut homodimers, unlike its human homologue. SEPT7 forms salt bridges which stabilises its G-interface. This is highlighted in Figure 1 below.
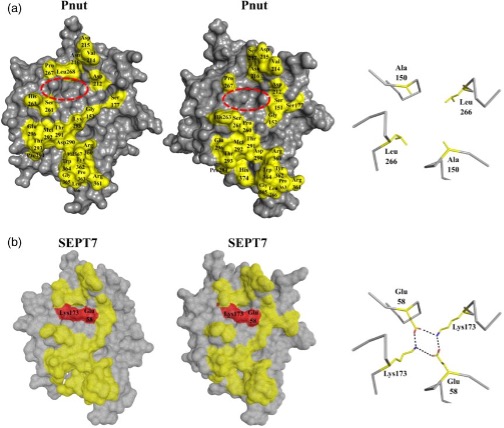
Figure 1: SEPT7 electrostatic region displayed in red, which is absent in Pnut (red circle)
A mutated Pnut with the necessary charged residues to form these bridges was expressed, and its own G-interface was stabilised to form homodimers, however these were weaker interactions. This may explain why Pnut tended to purify as a monomer rather than as a dimer, unlike SEPT7.
The study hoped to fill several gaps in the understanding of septin structure and conservation across evolution. Identification of the conservation of the G-interface, particularly in Sep1.Sep2 interactions was critical, but also the importance of salt bridge formation – showcased by its absence in Pnut.
Read the full study here - De Freitas Fernandes et al (2022)